OUR PIPELINE
Focused on Highly Underserved Markets
Noema Pharma is advancing three parallel programs across both orphan and large-market neurology indications with substantial unmet need. Our late-stage candidates are designed to improve the lives of people with trigeminal neuralgia, Tourette syndrome, and vasomotor symptoms (hot flashes & night sweats), as well as other symptoms of menopause.
Our Pipeline
Click an asset below to learn more.
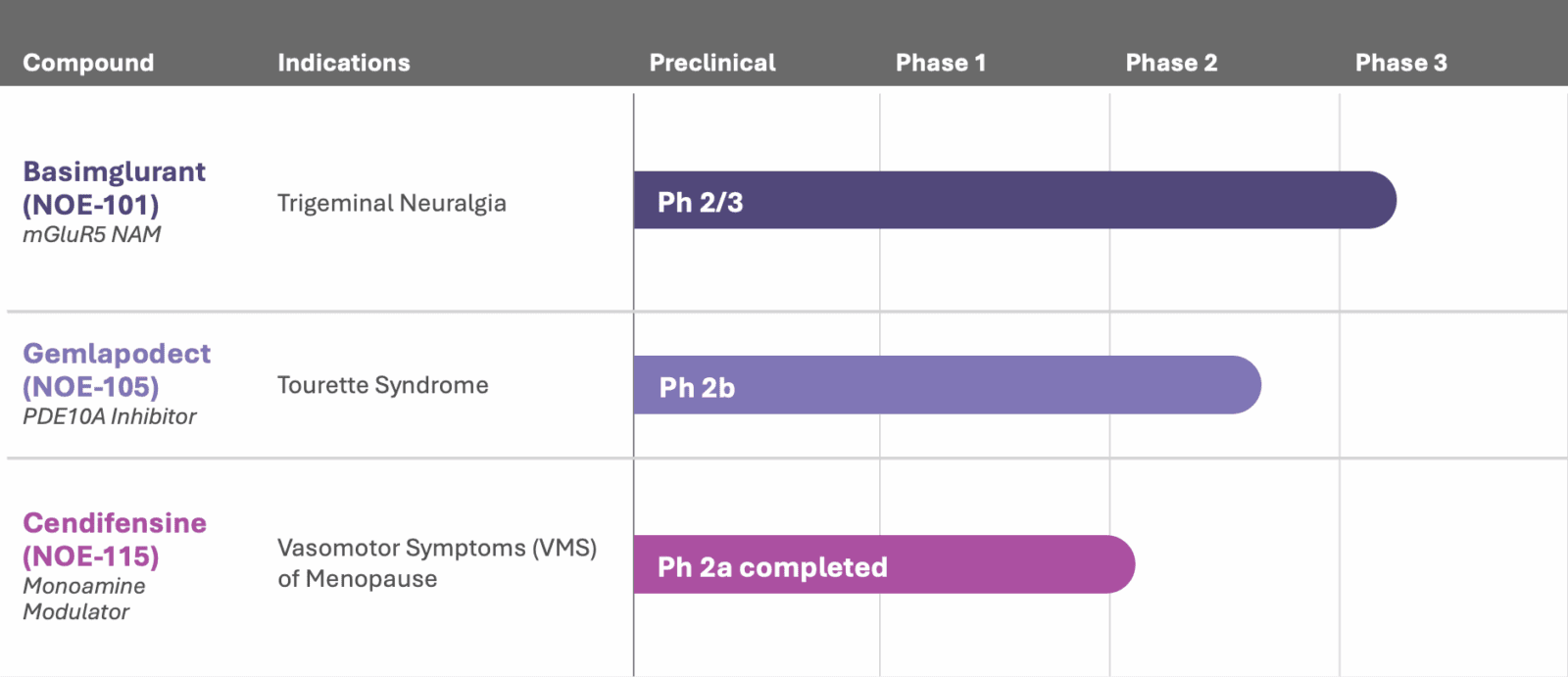
mGluR2/3 NAM, negative allosteric modulator of mGlu2/3 receptors; mGluR5 NAM, negative allosteric modulator of mGlu5 receptor; PDE10Ai, phosphodiesterase 10A inhibitor; TN, trigeminal neuralgia; COFD, Childhood Onset Fluency Disorder.
*Under indication validation.
Basimglurant
(NOE-101)
Potential First-in-Class mGluR5 NAM
For the management of pain associated with Trigeminal Neuralgia (TN)
Clinical stage: Phase 2/3

Basimglurant (NOE-101) is a potential first-in-class, highly selective, and potent mGluR5 negative allosteric modulator designed to provide robust neuropathic pain control in TN. Its well-characterized mechanism impacts both central and peripheral pain pathways and enables effective inhibition of intracellular and nuclear mGluR5 receptors implicated in neuropathic pain.
Basimglurant has demonstrated strong target engagement, the highest cellular permeability among mGluR5 inhibitors, and a favorable tolerability profile in over 1,000 subjects, with no known abuse potential. The program has received U.S. FDA Fast Track designation.
Expanded Access / Early Access Policy: Basimglurant - NOE-101
Version: 01 Nov 2022
Drugs being studied in clinical trials have unknown benefits and unknown risks that will not be understood until the clinical trials are complete.
Eventually, if results from the clinical trials are favorable, they will be submitted to FDA and other regulatory bodies for review of the drug’s safety and efficacy in order to seek approval for the drug candidate. Obtaining regulatory approval for a new medicine is the best way to bring rapid access to the greatest number of patients who may benefit.
Sometimes patients may be able to access investigational drugs outside of a clinical trial. In the United States, this is possible through “expanded access,” sometimes also referred to as “compassionate use”. Unlike the use of an investigational drug in a clinical trial setting, the primary purpose of expanded access is to use the investigational drug for treatment purposes, rather than to gather data on safety and effectiveness.
In the early stage of drug development, it is critical to systematically obtain information about the safety and tolerability of the investigational drug in a controlled manner. It is also important to evaluate investigational drugs under controlled conditions to assess their efficacy. Sufficient data on safety, tolerability and effectiveness is not yet available for Noema’s compounds currently in development to allow an Expanded Access or Early Access Program. Therefore, Noema Pharma is not accepting expanded/early access requests at this time. We will reevaluate this policy in the future as information from our clinical trials become available. If the policy changes, this webpage will be updated.
If you have questions about this policy or would like information about how to enroll in our clinical studies, please contact us. You can also obtain information about our studies at https://clinicaltrials.gov/.
Gemlapodect
(NOE-105)
Potential First-in-Class PDE10A Inhibitor
For the management of Tourette Syndrome (TS) tics
Clinical stage: Phase 2b

Gemlapodect (NOE-105) is a potential first-in-class, potent, and selective PDE10A inhibitor that offers a mechanistically precise approach for the treatment of TS. By enhancing cAMP/cGMP signaling downstream of D1/D2 pathways and modulating subcortical dopamine activity intracellularly, it acts distinctly from current D1/D2 antagonists, which block dopamine receptors at the cell membrane. This differentiated intracellular mechanism has been shown to reduce dopamine-driven motor behaviors across multiple preclinical models, supporting its potential as a well-tolerated and superior treatment option for TS. Gemlapodect has previously demonstrated a favorable safety and tolerability profile in adult subjects.
Cendifensine (NOE-115)
Broad Spectrum Monoamine Modulator
For the treatment of vasomotor symptoms (VMS) due to
menopause
Clinical stage: Phase 2a
Cendifensine (NOE-115) is a broad-spectrum monoamine modulator designed as a non-hormonal treatment for menopause-related VMS (hot flashes & night sweats) and other menopause-related symptoms. By rebalancing key neurotransmitters—serotonin, norepinephrine, and dopamine—it addresses the thermoregulatory dysfunction that drives hot flashes and may also impact additional cortical and hypothalamic pathways disrupted during menopause.
In a Phase 2b clinical study, cendifensine demonstrated a favorable safety profile and encouraging efficacy, showing meaningful reductions in VMS frequency and severity along with improvements in mood, food cravings, weight, and sleep/fatigue.
